A quartz crystal is an electronic component that can generate arbitrary and stable frequencies thanks to the high Q factor of a crystal blank. However, when particles adhere to the surface of the crystal blank, this high Q factor causes a considerable increase of CI characteristics, because the particles impede the vibrations of the blank attributable to the piezoelectric property of the material. The resonator may cease to oscillate in some cases if its CI characteristics increase and its specific CI value becomes unavailable.
In this article, we present Murata’s original particle screening technology that ensures the screening of defective products, to which particles responsible for the deterioration of the characteristics of quartz crystals have adhered, during the production process.
2. A Particle Problem in Quartz Crystals
When particles adhere to the surface of a crystal blank, the vibration of the blank is impeded and the CI value increases as shown in Fig. 2, leading to the cessation of oscillation. Our research on the effect of particles on quartz crystals has revealed three contributing factors to the changes in CI characteristics: (1) the adhesion site of particles over a crystal blank, (2) the type of particles, and (3) the adherence (the state or quality of adhering) of particles.
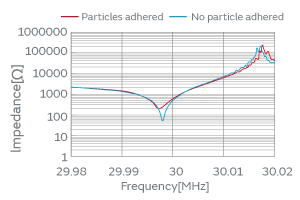
Fig. 2. Variation of the Characteristics before/after the Particle Adhesion
(1) The Effect of the Adhesion Site of Particles over a Crystal Blank
Particles were deliberately adhered to a crystal blank, and the effect of the adhesion site of the particles on the CI characteristics was investigated. The result indicated a great effect in the middle of the crystal blank as shown in Fig. 3. The fact that the vibrational displacement is the greatest in the middle of the crystal blank suggests that the vibration in the middle is susceptible to a load of mass, such as particles.
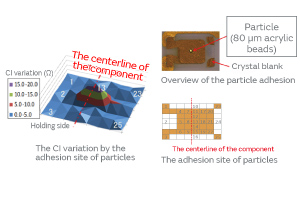
Fig. 3. The Effect of the Adhesion Site of Particles on the CI Characteristics
(2) The Effect of the Type of Particles
There are two kinds of particles: organic and inorganic. Examples of organic particles include those derived from the human body and fiber dust. An example of an inorganic particle is electrode dust. Both types of particles exist in a non-negligible amount in any production process. The organic and inorganic particles were deposited on a crystal blank, and the effect of each on the CI characteristics was investigated. Figure 4 shows the result. In the experiment, the CI value increase owing to the inorganic particles was smaller than that owing to the organic particles. We conjecture that the small contact surface area between the inorganic particles and the crystal blank impeded the propagation of the standing wave of the crystal.
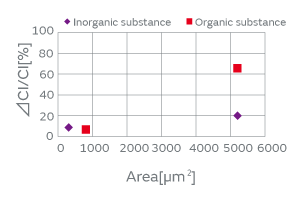
Fig. 4. The Effect of the Type of Particles on the CI Characteristics
(3) The Effect of the Adherence of Particles
When particles adhere to the surface of a crystal blank, how do the contact surface area and the adherence of particles affect the CI characteristics? We investigated to find an answer to this question. The effect on the CI characteristics was examined in the following two cases: In one case, the intact particles were deposited on a crystal blank, and in the other case, the particles were crushed before being deposited, that is, the adherence of the particles was altered. When the particles were crushed and thereby the contact surface area to the crystal blank was increased, the CI value was found to increase considerably (Fig. 5). The change in the CI value of the inorganic particles was found to be less than that of the organic particles. This can be accounted for by the smaller change of adhesiveness in the inorganic particles because of their structural resistance to deformation. The CI value changed about 10% in the inorganic particles. This can be explained by a small change in the contact state that probably occurred when a trace of organic particles adhered to the surface of the inorganic particles was crushed. The adherence of the organic particles is illustrated in Fig. 6. In order to investigate the effect of the adhesiveness of the particles on the crystal blank, the simulation was conducted by changing the Young’s modulus of the contact surface between the particles and the crystal blank. Figure 7 shows the relationship between the Young’s modulus and the CI variation. The higher the Young’s modulus of the contact surface, the greater the impact on the CI values. This relationship can be explained by the following: The higher the adhesiveness, that is, the higher the Young’s modulus of the contact surface, the easier the transmission of the vibration of the crystal to the particles. When the vibration of the crystal is transmitted to the particles, the damping term (1/Qm) of the particles starts to dominate the other terms, thereby impeding the vibration and increasing the CI value. When the Young’s modulus goes beyond a certain level, almost all of the vibration will be transmitted to the particles; hence, the CI value will approach a constant value dependent on the damping term of the particles. Because the impact of the particles is substantial as mentioned above, the over-excitation process is included before the cap sealing process in our production process for quartz crystals as shown in Fig. 8. The over-excitation process is a process that shakes off the particles from the crystal blank by applying high-power signals to the crystal blank and thereby forcing the blank to vibrate. The over-excitation process is effective in shaking off the particles in the middle of the crystal blank where the vibrational displacement of the blank is large, but it is not effective in the region where the vibrational displacement is small; in the latter region, the over-excitation process cannot shake off the particles sufficiently. Hence, in order to separate the products with the remaining particles that the over-excitation process failed to remove, the high-temperature treatment process is sequenced after the cap sealing process. The high-temperature treatment process makes the particles adhere to the crystal blank by exposing the products to a high-temperature environment.
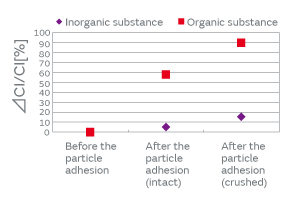
Fig. 5. The Effect of the Adherence of Particles on the CI Characteristics
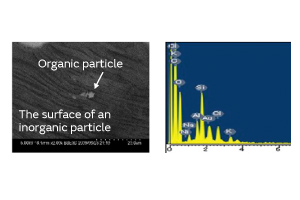
Fig. 6. Surface Analysis of an Inorganic Particle
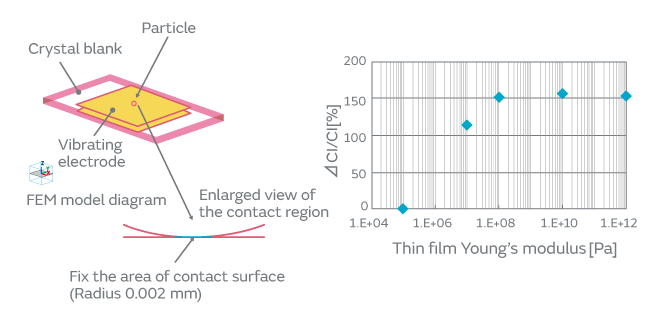
Fig. 7. The Effect of the Variation of Young’s Modulus of the Contact Surface
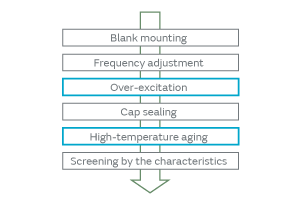
Fig. 8. Process Sequence
4. Solution to the Adhesion of Inorganic Particles
Because the surface of the inorganic particles contains a trace of organic material, we investigated the possibility of adhering the inorganic particles to a crystal blank using moisture (water molecules) as a mediator as illustrated in Fig. 10.
The products that incorporated a crystal blank, to whose surface particles were deliberately adhered, were left under a high-temperature and high-humidity environment, and the variation of the characteristics was investigated. The result indicated the effect of the inorganic particles on the CI value increase under the high-temperature and high-humidity environment. Figure 11 presents the CI variation under the following three different high-temperature and high-humidity environments: (a) at 125°C and a relative humidity (RH) of 40% for 24 hours, (b) at 180°C and 20% RH for 24 hours, and (c) at 85°C and 85% RH for 24 hours. The particles derived from the human body such as skin (organic substance A) are denoted by the symbol ◆, those derived from a cured adhesive substance in a form of minute fragments (organic substance B) are denoted by ■, and the inorganic particles derived from the powder of metal such as stainless steel (inorganic substance) are denoted by X. The plot under the (a) conditions indicates the CI variation was less than 50% in all cases. In particular, the blank to which the cured-adhesive-derived fragments (organic substance B) adhered and the blank to which the metal powder (inorganic substance) adhered exhibited a small CI variation. The plot under the (b) conditions shows the CI variation of the product to which the cured-adhesive-derived fragments (organic substance B) adhered was larger than that under (a), whereas the CI variation of the product to which metal powder (inorganic substance) adhered was not very large. In contrast, the plot under the (c) conditions shows that the CI variation was significantly large in all of the products to which the human-body-derived particles (organic substance A), the cured-adhesive-derived fragments (organic substance B), or the metal powder (inorganic substance) adhered.
The above result tells us that the high-temperature environment affects the CI characteristics of the organic particles, whereas the same environment affects those of the inorganic particles only slightly. Our humidity treatment deposits the products under a high-temperature and high-humidity environment to enhance the adhesiveness of the inorganic particles, thereby separating the particle-adhered products from the rest.
HCR has a resin-sealed structure, which allows the permeation of water vapor. The product deliberately allows water vapor to enter the package and lets moisture (water molecules) adsorb on the surface of the inorganic particles, which enhances the adhesiveness of the inorganic particles to the crystal blank. When an excess volume of water vapor enters the package, the load of the mass of moisture promotes the deterioration of the characteristics; therefore, the volume of water vapor entering the package must be controlled. The volume of water vapor that a package can hold, given by Equation 1, is dependent on the internal volume of the package. Figure 12 shows the relationship between the water content entering the package and the CI variation when the internal volume of the package was deliberately changed. For example, designing the internal volume of the HCR XRCHA series to make the water content no more than 220 ng allows you to verify no impact of the water content on the CI characteristics. To summarize the above, the introduction of the humidity treatment will ensure more secure screening of particles than the conventional high-temperature treatment.
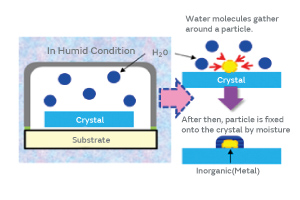
Fig. 10. The Mechanism of Moisture-Mediated Particle Adhesion
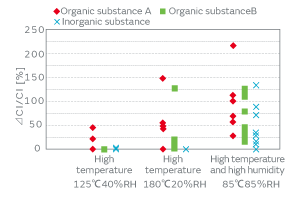
Fig. 11. The Effect of the Conditions of Aging on the CI Characteristics
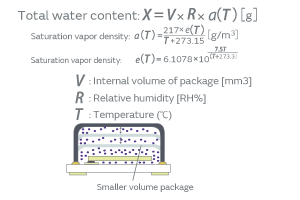
Equation 1. Water Content in a Package
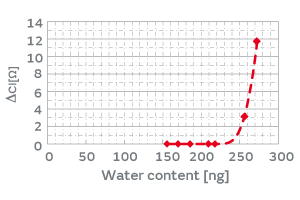
Fig. 12. The Effect of Water Content in the Product Package on the CI Characteristics